Frequently Asked Questions
JUMP TO SECTION:
JUMP TO SECTION:
ZYNRELEF is the first and only extended-release dual-acting local anesthetic.1-4 Its unique formulation combines the local anesthetic bupivacaine with a low dose of the NSAID meloxicam and was designed to overcome the challenges of inflammation at the surgical site. The meloxicam inhibits inflammation, normalizing pH at the surgical site and potentiating the effects of bupivacaine.1,5
Learn more about the dual-acting mechanism of action of ZYNRELEF.There have been no head-to-head studies of ZYNRELEF versus other available extended-release products or local anesthetic “cocktails” that combine bupivacaine with other medications. ZYNRELEF was tested against standard-of-care (SOC) bupivacaine HCl solution.1-3
Learn more about the dual-acting mechanism of action of ZYNRELEF.No. ZYNRELEF is the first and only extended-release dual-acting local anesthetic.1-4 It is also the only local anesthetic considered by FDA to be extended-release, based on superiority to bupivacaine through 72 hours.1 It combines bupivacaine with a low dose of meloxicam and is designed to overcome the challenges of inflammation at the surgical site, which creates an acidic environment that would otherwise make it difficult for bupivacaine to penetrate the nerve cell membrane and reach the intended site of action.1,5,6 ZYNRELEF uses proprietary Biochronomer® controlled-diffusion technology, a polymer that allows for delivery of the active ingredients over the course of 72 hours after surgery, providing sustained pain relief.1,5
Learn more about the dual-acting mechanism of action of ZYNRELEF.The inflammatory process associated with surgical incision creates an acidic environment that makes it difficult for local anesthetics to penetrate the nerve cell membrane and reach the intended site of action.6 Meloxicam is a potent NSAID with a long half-life, and it was selected specifically for its anti-inflammatory mechanism of action.1,7 The physicochemical properties allow it to be formulated into the polymer and have the intended release rates.7 The meloxicam in ZYNRELEF is thought to overcome the challenge of tissue acidosis caused by inflammation at the surgical site, normalizing pH at the surgical site in order to potentiate the analgesic effect of bupivacaine.1,5
Learn more about the dual-acting mechanism of action of ZYNRELEF.The active ingredients in ZYNRELEF are released using a proprietary Biochronomer controlled-diffusion technology.1,5
aReflects in vitro release rates of active ingredients.
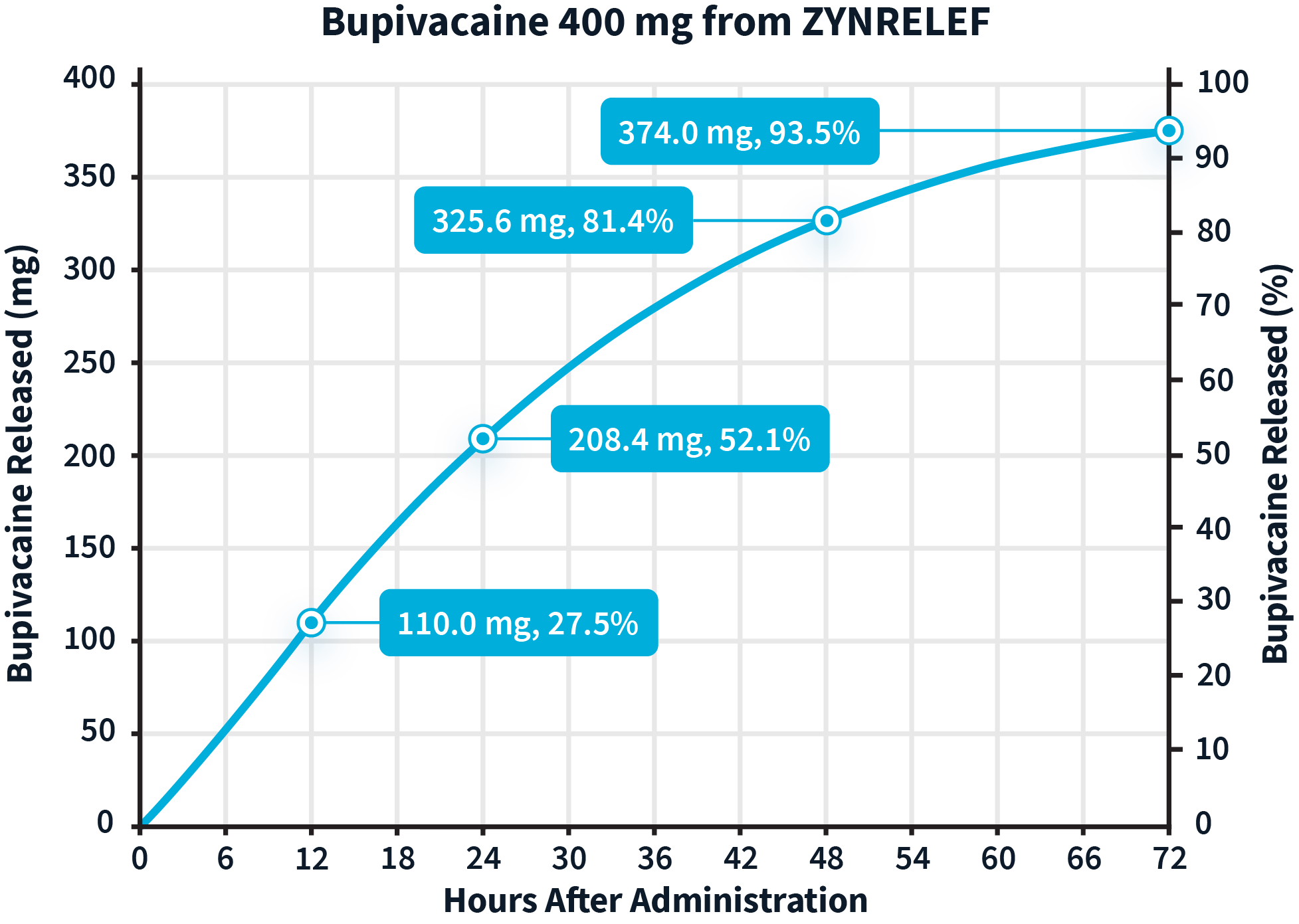
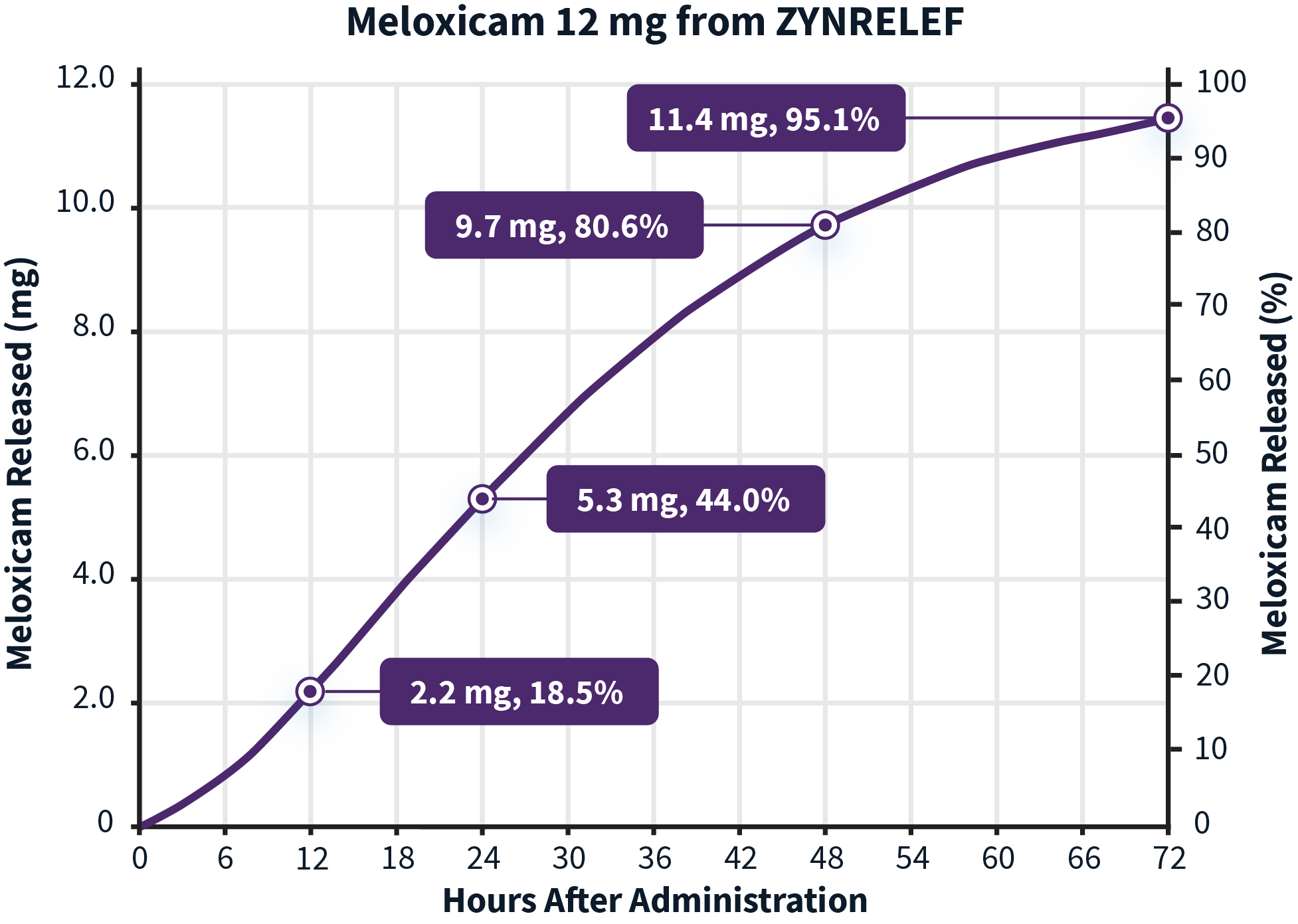
The inflammatory process associated with surgery creates an acidic environment that makes it difficult for local anesthetics to penetrate the nerve cell membrane and reach the intended site of action.6 Meloxicam is a potent NSAID with a long half-life, and was selected specifically for its anti-inflammatory mechanism of action.1,7 It is thought to work synergistically to reduce local inflammation, thereby potentiating the analgesic efficacy of bupivacaine.1
Learn more about the dual-acting mechanism of action of ZYNRELEF.Due to the novel properties of the Biochronomer technology, lidocaine and other local anesthetics can be administered before ZYNRELEF without causing ZYNRELEF to “dump” the active ingredients all at once. Avoid additional use of local anesthetics within 96 hours following administration of ZYNRELEF. ZYNRELEF should be applied as is, without diluting or mixing with water, saline, or other local anesthetics. No mixing with bupivacaine is required to achieve efficacy with ZYNRELEF.1
Learn more about the dual-acting mechanism of action of ZYNRELEF.ZYNRELEF is available through full-line wholesalers and specialty distributors; prime vendor discounts apply. GPO and sub-WAC pricing are also available. In addition, reimbursement for ZYNRELEF may offset the cost of the product for some patients.
| ZYNRELEF Pricing | ||
|---|---|---|
| Volume | Bupivacaine/Meloxicam | WAC |
| 14 mL | 400 mg/12 mg | $317.40 |
| 7 mL | 200 mg/6 mg | $158.70 |
GPO: group purchasing organization. WAC: wholesaler acquisition cost.
ZYNRELEF pricing as of January 1, 2025. Confirm current pricing with your Heron representative.
Learn more about pricing and reimbursement for ZYNRELEF.ZYNRELEF is room temperature stable for 36 months.9 The vial should be stored at a controlled room temperature (20°C to 25°C; 68°F to 77°F). Temperature excursions are permitted within 15°C to 30°C (59°F to 86°F). Product should be used at room temperature.1
Learn more about ZYNRELEF dosing and administration.Yes. ZYNRELEF is room temperature stable,1 and the kit will fit into automated dispensing systems.
Learn more about ZYNRELEF dosing and administration.ZYNRELEF is available in 2 different vial amounts:1
As a general guidance in selecting the proper dosing of ZYNRELEF, the following examples of dosing are provided1:
Limit exposure to articular cartilage due to the potential risk of chondrolysis.
Note: Not all procedures listed under each vial size require full contents to cover affected tissues.
Learn more about ZYNRELEF dosing and administration.ZYNRELEF spreads easily and covers a large area. The 14-mL dose volume is sufficient to cover total knee arthroplasty (TKA), a large surgical procedure.1
Learn more about ZYNRELEF dosing and administration.The extraction site of a laparoscopic procedure is a small-to-medium open wound, which means that with the approval of the recent sNDA in December 2021, ZYNRELEF is now indicated for laparoscopic procedures. When applying ZYNRELEF during a laparoscopic procedure, ZYNRELEF can be placed onto the fascia of the trocar and extraction sites. It should not be applied directly to the peritoneum. Prior to application, it is recommended to add a stitch within each port site to keep the product in place on pain-generating tissues.1
Learn more about ZYNRELEF dosing and administration.The active ingredients of ZYNRELEF diffuse throughout the affected tissue at the surgical site. Meloxicam is thought to potentiate the effect of bupivacaine by facilitating penetration into the nerve cell at the intended site of action so that the bupivacaine can block pain signals to the brain.1,5,6
Learn more about ZYNRELEF dosing and administration.ZYNRELEF is a viscous formulation and does not require volume expansion to achieve efficacy. Therefore, the product should not be mixed with water, saline, or other local anesthetics. However, other local anesthetics such as lidocaine can be administered before ZYNRELEF without causing release of the active ingredients all at once.1
Learn more about ZYNRELEF dosing and administration.The maximum amount of meloxicam in ZYNRELEF is 12 mg released over 3 days.1
Heron Therapeutics, Inc, has investigated the use of ZYNRELEF as part of a multimodal analgesic strategy. In cohorts receiving ketorolac, celecoxib, or ibuprofen in addition to ZYNRELEF, no increase in NSAID-related toxicity was seen.10-14
Following administration of ZYNRELEF, if additional NSAID medication is indicated in the postoperative period, monitor patients for signs and symptoms of NSAID-related GI adverse reactions.1
In the ZYNRELEF clinical development program, there was no evidence of LAST based on a review of potential LAST-related treatment-emergent adverse events (TEAEs), vital signs, ECGs, and bupivacaine plasma concentrations.14,15
As part of the clinical research program for ZYNRELEF, a summary of LAST assessment questionnaire results was generated, showing the number and percentage of subjects with any potential signs or symptoms of LAST, with a breakdown of each sign or symptom overall and by each visit.15-17 The proportion of patients treated with ZYNRELEF who had a potential LAST-related TEAE was lower than that of standard bupivacaine and similar to that of placebo (ie, subjects with no exposure to bupivacaine). Additionally, despite the extended release of bupivacaine from ZYNRELEF over 3 days, the incidences of potential LAST-related TEAEs with a rapid onset (within 1 hour after study drug administration) were similar across all treatment groups. The incidences of potential LAST-related TEAEs with a delayed onset (between 1 to 72 hours after study drug administration) in the ZYNRELEF group were lower than the standard bupivacaine group and similar to placebo group.15
View the ZYNRELEF full Prescribing Information for more LAST-related warnings and precautions.NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events.
No events of gastrointestinal bleeding or perforation that were reported in any of the clinical trials were related to treatment with ZYNRELEF.11,14,15,18-21 Even in cohorts receiving ketorolac, celecoxib, or ibuprofen in addition to ZYNRELEF, no increase in NSAID-related toxicity was seen with this one-time low dose of meloxicam released over 3 days.10,11,13,14
Following administration of ZYNRELEF, if additional NSAID medication is indicated in the postoperative period, monitor patients for signs and symptoms of NSAID-related GI adverse reactions.
The highest recommended dose of ZYNRELEF contains 400 mg bupivacaine and a low dose (12 mg) of meloxicam, released over 72 hours.1
Only subjects with a creatinine clearance of >30 mL/min were considered eligible to participate. Therefore, there is no data on patients with severe renal impairment.16-20, 22-26
Bupivacaine and meloxicam and their metabolites are excreted by the kidney. No dose adjustment of ZYNRELEF is necessary in patients with mild to moderate renal impairment. Patients with renal disease, especially those with severe renal disease, may be more susceptible to the potential toxicities of the amide-type local anesthetics. Patients with severe renal impairment have not been studied. The use of ZYNRELEF in patients with severe renal impairment is not recommended. Meloxicam is not dialyzable. When using ZYNRELEF in patients on hemodialysis do not exceed maximum recommended dose or use with other meloxicam-containing products.1
Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of ZYNRELEF in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal function.1
In adequate and well-controlled trials, patients treated with ZYNRELEF did not experience increased rates of infection.15
Do not inject ZYNRELEF.
The polymer in ZYNRELEF has a high viscosity1 that renders it extremely difficult to push through even a large hypodermic needle. While this practical barrier does not remove the risk of attaching a needle to the syringe, it greatly reduces the risk of someone trying to administer ZYNRELEF as an injection, and the in-market packaging will include pronounced warnings against the use of any Luer lock attachment other than the provided applicator.
Visit the Resource Center for cards and videos that can help you administer ZYNRELEF.Compatibility with ZYNRELEF was tested with Prolene® mesh, silicone membranes, bone cement, and metal alloys used in surgical implants. ZYNRELEF is also compatible with all components of the ZYNRELEF kit, including syringes, Luer lock cone-shaped applicator, vented vial spike, and syringe tip caps.1
See more safety information for ZYNRELEF.ZYNRELEF is not indicated for nerve block, as Heron Therapeutics, Inc, is focused on administration by needle-free instillation into the surgical site.1
Yes, generic bupivacaine HCl solution or ropivacaine as a nerve block may be used. However, ZYNRELEF has not been studied using block anesthesia and is contraindicated for use in patients undergoing obstetrical paracervical block anesthesia. It also should not be used in obstetrical procedures prior to delivery. Nerve blocks using longer-acting local anesthetics (liposomal bupivacaine) are not necessary because ZYNRELEF provides up to 72 hours of analgesia.1
The patient’s total exposure to local anesthetics must be considered before application of ZYNRELEF, as well as during the first 72 hours that follow application. Avoid additional use of local anesthetics within 96 hours following administration of ZYNRELEF.1
Release rates of the active ingredients8,a:
aReflect in vitro release rates of active ingredients.
View the ZYNRELEF full Prescribing Information to see the full list of warnings and precautions.
ZYNRELEF has been studied in multiple surgical models, including bunionectomy, herniorrhaphy, and total knee arthroplasty.1
Learn more about the efficacy of ZYNRELEF.Registering will put you at the front of the line to receive updates on the availability of ZYNRELEF and tools/resources.
ZYNRELEF is indicated in adults for instillation to produce postsurgical analgesia for up to 72 hours after soft tissue and orthopedic procedures including foot and ankle, and other procedures in which direct exposure to articular cartilage is avoided.
Limitations of Use: Safety and efficacy have not been established in highly vascular surgeries, such as intrathoracic, large 4 or more level spinal, and head and neck procedures.
ZYNRELEF is contraindicated in patients with a known hypersensitivity (eg, anaphylactic reactions and serious skin reactions) to any amide local anesthetic, NSAIDs, or other components of ZYNRELEF; with history of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients); undergoing obstetrical paracervical block anesthesia; or undergoing CABG.
Dose-Related Toxicity: Monitor cardiovascular and respiratory vital signs and patient’s state of consciousness after application of ZYNRELEF. When using ZYNRELEF with other local anesthetics, overall local anesthetic exposure must be considered through 72 hours.
Hepatotoxicity: If abnormal liver tests persist or worsen, perform a clinical evaluation of the patient.
Hypertension: Patients taking some antihypertensive medication may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure.
Heart Failure and Edema: Avoid use of ZYNRELEF in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure.
Renal Toxicity: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of ZYNRELEF in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal failure.
Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs.
Risk of Joint Cartilage Necrosis and Degeneration with Unapproved Intra-articular Use: Animal studies evaluating the effects of ZYNRELEF following intra-articular administration in the knee joint demonstrated cartilage necrosis and degeneration.
Chondrolysis: Limit exposure to articular cartilage due to the potential risk of chondrolysis.
Methemoglobinemia: Cases have been reported with local anesthetic use.
Serious Skin Reactions: NSAIDs, including meloxicam, can cause serious skin adverse reactions. NSAIDs can also cause fixed drug eruption (FDE). FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. If symptoms present, evaluate clinically.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): If symptoms are present, evaluate clinically.
Fetal Toxicity: Due to the risk of oligohydramnios/fetal renal dysfunction and premature closure of the ductus arteriosus with NSAIDs, limit use of ZYNRELEF between about 20 to 30 weeks gestation, and avoid use after about 30 weeks.
Hematologic Toxicity: Monitor hemoglobin and hematocrit in patients with any signs or symptoms of anemia.
Drugs That Interfere with Hemostasis: Monitor patients for bleeding who are using ZYNRELEF with drugs that interfere with hemostasis (eg, warfarin, aspirin, SSRIs/SNRIs).
ACE Inhibitors, Angiotensin Receptor Blockers (ARBs), or Beta-Blockers: Use with ZYNRELEF may diminish the antihypertensive effect of these drugs. Monitor blood pressure.
ACE Inhibitors and ARBs: Use with ZYNRELEF in elderly, volume-depleted, or those with renal impairment may result in deterioration of renal function. In such high-risk patients, monitor for signs of worsening renal function.
Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects.
Infertility: NSAIDs are associated with reversible infertility. Consider avoidance of ZYNRELEF in women who have difficulties conceiving.
Severe Hepatic Impairment: Only use if benefits are expected to outweigh risks; monitor for signs of worsening liver function.
Severe Renal Impairment: Not recommended.
Most common adverse reactions (incidence ≥5%) in controlled clinical trials with ZYNRELEF are soft tissue procedures: vomiting and orthopedic procedures: constipation and headache.
Report side effects to Heron at 1-844-437-6611 or to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ZYNRELEF is indicated in adults for instillation to produce postsurgical analgesia for up to 72 hours after soft tissue and orthopedic procedures including foot and ankle, and other procedures in which direct exposure to articular cartilage is avoided.
Limitations of Use: Safety and efficacy have not been established in highly vascular surgeries, such as intrathoracic, large 4 or more level spinal, and head and neck procedures.
Please see full Prescribing Information, including Boxed Warning and updated Warnings and Precautions for serious skin reactions caused by nonsteroidal anti-inflammatory drugs (NSAIDs).
How could you incorporate ZYNRELEF as the foundation of your institution’s postoperative pain management strategy? Connect with us to find out.
Fields marked with an asterisk (*) are required.
In the meantime, you can learn more about ZYNRELEF by viewing our Frequently Asked Questions or downloading our Resources.
844-HERON11 (844-437-6611)