ZYNRELEF is indicated in adults for instillation to produce postsurgical analgesia for up to 72 hours after soft tissue and orthopedic procedures including foot and ankle, and other procedures in which direct exposure to articular cartilage is avoided.
Limitations of Use: Safety and efficacy have not been established in highly vascular surgeries, such as intrathoracic, large 4 or more level spinal, and head and neck procedures.
72 hours of pain relief and opioid reduction in soft tissue surgery1-4
Greater pain reduction in herniorrhaphy trials
Patients treated with ZYNRELEF showed a 21% reduction in pain intensity compared to those treated with bupivacaine HCl solution.2
51% of ZYNRELEF patients experienced no severe pain in the first 72 hours—a 30% increase over patients treated with bupivacaine HCl solution.2
More opioid-free patients in herniorrhaphy trials
51% of patients treated with ZYNRELEF required no opioids in the first 72 hours following surgery, compared with 40% of patients treated with bupivacaine HCl solution.1,2
Of ZYNRELEF patients who were opioid-free at 72 hours, 85% remained opioid-free through day 28.2
91% of patients required no opioids through 72 hours when treated with ZYNRELEF plus a scheduled, non-opioid, multimodal regimen of over-the-counter oral acetaminophen and ibuprofen.3
85% of patients remained opioid-free through day 28 when treated with ZYNRELEF plus the scheduled, non-opioid multimodal regimen.3
95% of patients treated with ZYNRELEF were opioid-free through day 15 when ZYNRELEF was used as the foundation of a non-opioid multimodal analgesic regimen in a real-world setting. In The HOPE Project, none of the 91% of patients who were discharged without an opioid prescription called back with postoperative pain.4
ZYNRELEF as the foundation of multimodal analgesia
Using ZYNRELEF in surgical protocols can:
- Reduce severe postoperative pain5
- Help prevent unnecessary exposure to opioids after surgery1-4
- Reduce or eliminate opioid prescriptions,1-4 reducing the opioid compliance burden on clinicians and staff
- Reduce opioid-related adverse events2,6
- Position healthcare organizations as part of the solution to the overuse of opioids in the surgical setting
- Reduce or eliminate callbacks from discharged patients4
Resources for you
Adding ZYNRELEF to formulary
Interested in seeing ZYNRELEF added to your institution’s formulary? Contact a Heron representative today to find out about becoming a champion for the next generation of postoperative pain management.
EPOCH 2 Herniorrhaphy Study Design
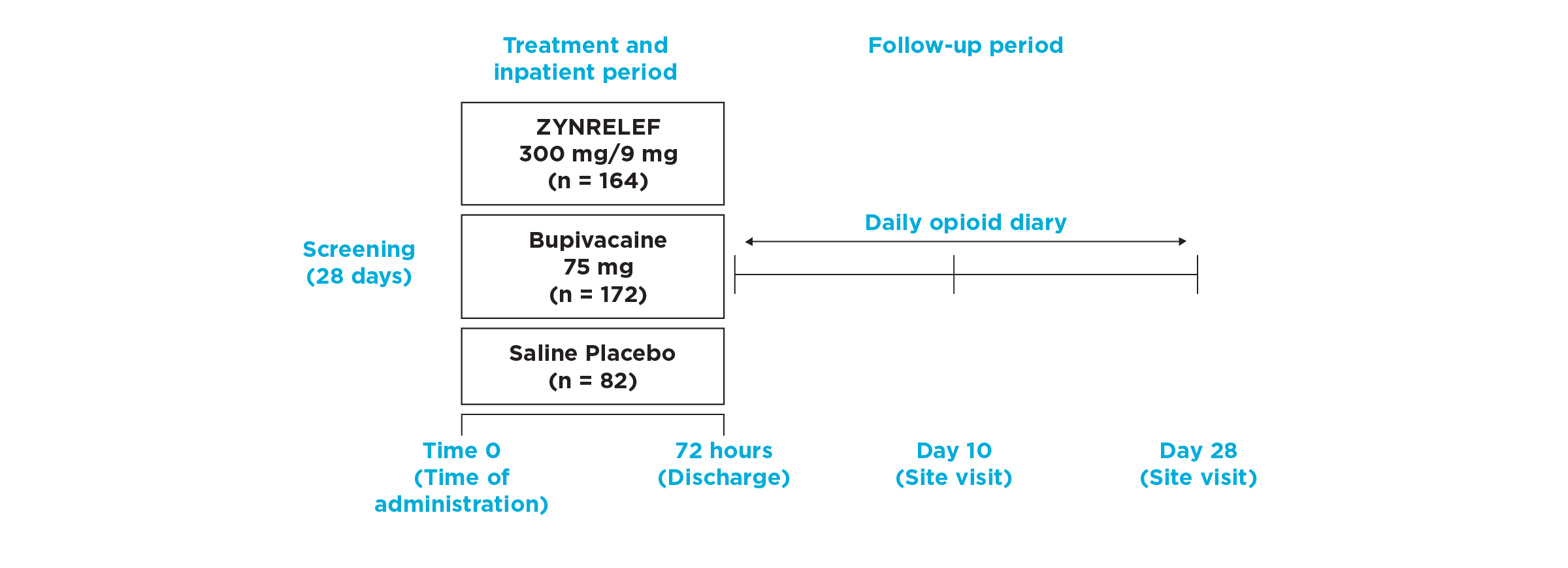
- The Phase 3, randomized, double-blind, multicenter EPOCH 2 trial shows how ZYNRELEF performed in patients who underwent open inguinal herniorrhaphy with mesh under general anesthesia. This trial compared ZYNRELEF not only to placebo but also to the standard of care, bupivacaine HCl solution. Of the 418 total patients in the intent-to-treat population, the mean patient age was 49 years (range, 18-83 years). Patients were predominantly male (94%).
- During surgery, intravenous fentanyl (≤4 μg/kg) was permitted for intraoperative pain control, and all patients were to receive an additional 50 μg intravenous fentanyl just prior to the end of surgery. No other opioids, analgesics, or anti-inflammatory agents (except study drug) were permitted intraoperatively, unless needed to treat an adverse event, for pretreatment prior to needle placement, or to decrease venous irritation.
- Per protocol, patients were to receive rescue medication only upon request for pain control during the 72-hour postoperative observation period. Medication was not permitted for pain prophylaxis. Upon request, patients requiring postoperative rescue medication could receive oral immediate-release oxycodone (≤10 mg within a 4-hour period as needed), intravenous morphine (≤10 mg within a 2-hour period as needed), and/or oral acetaminophen (≤1 g in a 6-hour period). For patients administered any acetaminophen-containing product, the total daily dose could not exceed 4 g. No other analgesic agents, including NSAIDs, were permitted during the 72-hour postoperative observation period.
- Following the 72-hour period, there were 10-day and 28-day follow-ups to assess opioid use over that period, as captured by patient diaries. Conservatively, opioid use was imputed to patients if on any day they missed a diary entry.
- Patients rated pain intensity on the 0-to-10 Numeric Rating Scale (NRS) of pain, and missing scores were imputed by carrying forward the last nonmissing postdose value. Pain scores were then analyzed with adjustment for the analgesic duration of rescue medications.
- Hierarchically tested endpoints were as follows:
- Primary: pain intensity area under the curve through 72 hours (AUC0-72) versus placebo
- First key secondary: pain intensity (AUC0-72) versus bupivacaine HCl solution
- Second key secondary: mean total opioid use (0-72 hours) versus placebo
- Third key secondary: proportion opioid-free (0-72 hours) versus bupivacaine HCl solution
- Fourth key secondary: mean total opioid use (0-72 hours) versus bupivacaine HCl solution
- Additional prespecified secondary endpoints (not controlled for multiple hypothesis testing) included:
- Pain intensity AUC0-12
- Pain intensity AUC0-24
- Pain intensity AUC0-48
- Proportion with any severe pain (NRS ≥7) through 72 hours
- Proportion opioid-free (0-72 hours) versus placebo
- Post hoc analyses included:
- Pain intensity AUC0-8
- Pain intensity AUC24-72
- Proportion opioid-free and without severe pain (NRS <7) through 72 hours
- Adverse events that emerged during this study were recorded through a safety follow-up visit on day 28. Patients who reported multiple events within a preferred term were counted only once for that preferred term. Opioid-related events were categorized by prespecified preferred terms as opioid related regardless of whether a patient actually received an opioid.
EPOCH 2 Single-Arm Follow-On Study Design
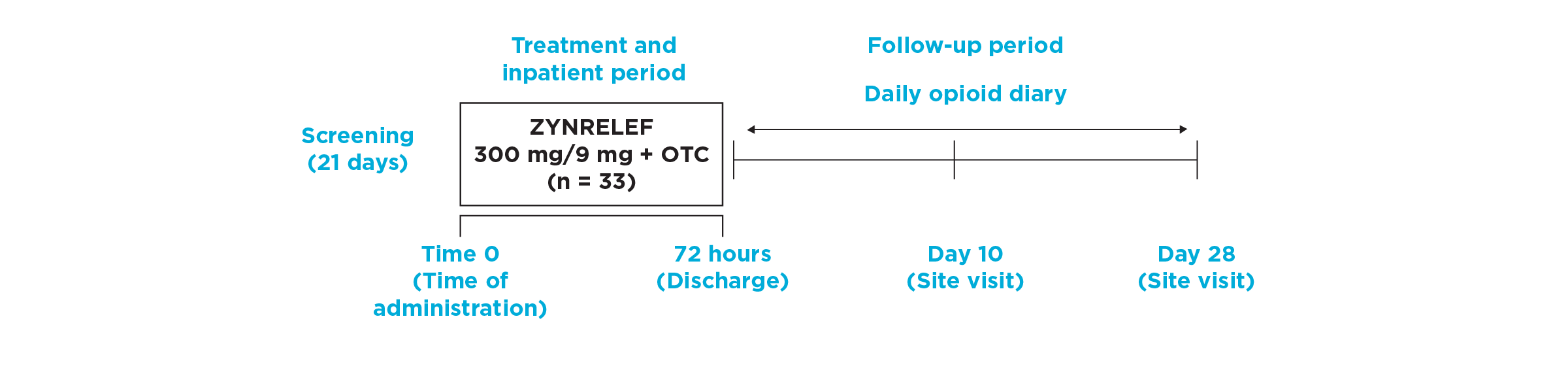
- In this Phase 2, open-label follow-on study to EPOCH 2, ZYNRELEF was used as the foundation of a scheduled multimodal analgesic regimen in patients who underwent open inguinal herniorrhaphy with mesh under general anesthesia. Compared to historical data from EPOCH 2, ZYNRELEF with a scheduled multimodal postoperative analgesic regimen was evaluated for reduction in mean pain intensity through 72 hours and the need for postoperative opioids through 72 hours and 28 days. ZYNRELEF was evaluated both with (n = 30) and without (n = 33) coadministration of intravenous ketorolac in addition to the postoperative multimodal analgesic regimen. Overall, the mean patient age was 49 years (range, 20-74 years), and patients were predominantly male (94%).
- During surgery, intravenous fentanyl (≤3 μg/kg) was permitted for intraoperative pain control, and all patients were to receive an additional 50 μg intravenous fentanyl just prior to the end of surgery. No other opioids, analgesics, or anti-inflammatory agents (except ZYNRELEF, with or without ketorolac) were permitted intraoperatively, unless needed to treat an adverse event, for pretreatment prior to needle placement, or to decrease venous irritation.
- Per protocol, patients were to receive opioid rescue medication only upon request for pain control during the 72-hour postoperative observation period. Rescue medication was not permitted for pain prophylaxis. Upon request, patients requiring postoperative rescue medication could receive oral immediate-release oxycodone (≤10 mg within a 4-hour period as needed) or intravenous morphine (≤10 mg within a 2-hour period as needed).
- On the day of surgery, patients received 1 g oral acetaminophen approximately 2 hours before general anesthetic induction. Following surgery, patients received a scheduled non-opioid multimodal postoperative analgesic regimen with oral ibuprofen and acetaminophen. The addition of 1 intraoperative dose of ketorolac provided no added benefit beyond oral ibuprofen and oral acetaminophen. Once patients were able to tolerate oral intake in the postoperative anesthesia care unit, they received 600 mg oral ibuprofen every 6 hours. Three hours after the first dose of ibuprofen, they started 1 g oral acetaminophen every 6 hours, alternating the 2 medications so that an analgesic was administered every 3 hours until the 72-hour inpatient postoperative period was complete.
- Following the 72-hour period, there were 10-day and 28-day follow-ups to assess opioid use over that period, as captured by patient diaries. Conservatively, opioid use was imputed to patients if on any day they missed a diary entry.
- Patients rated pain intensity on the 0-to-10 Numeric Rating Scale (NRS) of pain, and missing scores were imputed by carrying forward the last nonmissing postdose value. Pain scores were then analyzed with adjustment for the analgesic duration of rescue medications. (Note: EPOCH 2 follow-on pain intensity results reflect reported pain intensity at rest; EPOCH 2 results reflect reported pain intensity with activity, ie, after sitting up from a resting position.)
- Adverse events that emerged during this study were recorded through a safety follow-up visit on day 28. Patients who reported multiple events within a preferred term were counted only once for that preferred term. Opioid-related events were categorized by prespecified preferred terms as opioid related regardless of whether a patient actually received an opioid.
HOPE Hernia 1 Study Design
- In this Phase 3b, randomized, multicenter, open-label study, ZYNRELEF was used as the foundation of a scheduled multimodal analgesic (MMA) regimen in patients who underwent open inguinal herniorrhaphy with mesh under deep sedation or general anesthesia. Compared to EPOCH 2 and the EPOCH 2 follow-on, this 2-part study was designed to evaluate ZYNRELEF with a scheduled postoperative MMA regimen in a more real-world context: Instead of a mandatory 72-hour postoperative observation period, patients were discharged per site practice and were instructed to follow their assigned MMA regimen at home.
- The primary objective of Part 1 was to identify which of 2 postoperative non-opioid MMA regimens, together with intraoperative ZYNRELEF, resulted in the highest proportion of patients who did not require a prescription for postoperative opioids through a follow-up visit on postoperative day 15.
- The secondary objectives were to assess postdischarge opioid consumption (number of pills consumed, reported per patient recall on day 15) and to assess patient satisfaction with the postoperative MMA regimen (on day 15, using the 9-item Treatment Satisfaction Questionnaire for Medication). A total of 93 patients were randomized to the 2 cohorts; the mean patient age was 49.5 years (range, 24-73 years), and patients were predominantly male (99%).
- During surgery, intravenous fentanyl up to 3 μg/kg was permitted for intraoperative pain control. No other opioids, analgesics, or anti-inflammatory agents (except ZYNRELEF) were permitted intraoperatively, unless needed to treat an adverse event, for pretreatment prior to needle placement, or to decrease venous irritation.
- After surgery but prior to discharge, opioid rescue medication could be administered according to the institution’s standard of care and only upon request for pain control. Rescue medication was not permitted for pain prophylaxis.
- At the time of discharge, patients rated their postoperative pain intensity on the 0-to-10 Numeric Rating Scale (NRS) of pain. Only patients with an NRS pain intensity score of 6 or greater and/or who were administered a predischarge opioid could receive an opioid prescription at discharge. Per protocol, the prescription was to be for oxycodone (ten 5-mg pills) only and not permitting of any substitution. After discharge and until the follow-up visit on day 15, patients who contacted the site about postoperative pain could receive a prescription for oxycodone (ten 5-mg pills and no substitutions) at the investigator’s discretion. Thereafter, postoperative pain was to be addressed per institutional standard of care.
- On the day of surgery (day 1), patients received 1 g oral acetaminophen and 400 mg oral ibuprofen approximately 2 hours before induction of general anesthesia or deep sedation. Following surgery, patients received a scheduled non-opioid MMA regimen with oral ibuprofen and acetaminophen once they were able to tolerate oral intake:
- Cohort 1 (alternating regimen): Patients were instructed to take 600 mg oral ibuprofen every 6 hours. Three hours after the first dose of ibuprofen, they were to start 1 g oral acetaminophen every 6 hours, alternating the 2 medications so that an analgesic would be taken every 3 hours.
- Cohort 2 (concurrent regimen): Patients were instructed to take 600 mg oral ibuprofen and 1 g oral acetaminophen concurrently every 6 hours.
- Adverse events that emerged during this study were recorded through a safety follow-up visit on day 29.
Important Safety Information and Indication
Indication
ZYNRELEF is indicated in adults for instillation to produce postsurgical analgesia for up to 72 hours after soft tissue and orthopedic procedures including foot and ankle, and other procedures in which direct exposure to articular cartilage is avoided.
Limitations of Use: Safety and efficacy have not been established in highly vascular surgeries, such as intrathoracic, large 4 or more level spinal, and head and neck procedures.
Important Safety Information
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use.
- ZYNRELEF is contraindicated in the setting of coronary artery bypass graft (CABG) surgery.
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events.
Contraindications
ZYNRELEF is contraindicated in patients with a known hypersensitivity (eg, anaphylactic reactions and serious skin reactions) to any amide local anesthetic, NSAIDs, or other components of ZYNRELEF; with history of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients); undergoing obstetrical paracervical block anesthesia; or undergoing CABG.
Warnings and Precautions
Dose-Related Toxicity: Monitor cardiovascular and respiratory vital signs and patient’s state of consciousness after application of ZYNRELEF. When using ZYNRELEF with other local anesthetics, overall local anesthetic exposure must be considered through 72 hours.
Hepatotoxicity: If abnormal liver tests persist or worsen, perform a clinical evaluation of the patient.
Hypertension: Patients taking some antihypertensive medication may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure.
Heart Failure and Edema: Avoid use of ZYNRELEF in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure.
Renal Toxicity: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of ZYNRELEF in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal failure.
Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs.
Risk of Joint Cartilage Necrosis and Degeneration with Unapproved Intra-articular Use: Animal studies evaluating the effects of ZYNRELEF following intra-articular administration in the knee joint demonstrated cartilage necrosis and degeneration.
Chondrolysis: Limit exposure to articular cartilage due to the potential risk of chondrolysis.
Methemoglobinemia: Cases have been reported with local anesthetic use.
Serious Skin Reactions: NSAIDs, including meloxicam, can cause serious skin adverse reactions. NSAIDs can also cause fixed drug eruption (FDE). FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. If symptoms present, evaluate clinically.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): If symptoms are present, evaluate clinically.
Fetal Toxicity: Due to the risk of oligohydramnios/fetal renal dysfunction and premature closure of the ductus arteriosus with NSAIDs, limit use of ZYNRELEF between about 20 to 30 weeks gestation, and avoid use after about 30 weeks.
Hematologic Toxicity: Monitor hemoglobin and hematocrit in patients with any signs or symptoms of anemia.
Drug Interactions
Drugs That Interfere with Hemostasis: Monitor patients for bleeding who are using ZYNRELEF with drugs that interfere with hemostasis (eg, warfarin, aspirin, SSRIs/SNRIs).
ACE Inhibitors, Angiotensin Receptor Blockers (ARBs), or Beta-Blockers: Use with ZYNRELEF may diminish the antihypertensive effect of these drugs. Monitor blood pressure.
ACE Inhibitors and ARBs: Use with ZYNRELEF in elderly, volume-depleted, or those with renal impairment may result in deterioration of renal function. In such high-risk patients, monitor for signs of worsening renal function.
Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects.
Use in Specific Populations
Infertility: NSAIDs are associated with reversible infertility. Consider avoidance of ZYNRELEF in women who have difficulties conceiving.
Severe Hepatic Impairment: Only use if benefits are expected to outweigh risks; monitor for signs of worsening liver function.
Severe Renal Impairment: Not recommended.
Adverse Reactions
Most common adverse reactions (incidence ≥5%) in controlled clinical trials with ZYNRELEF are soft tissue procedures: vomiting and orthopedic procedures: constipation and headache.
Report side effects to Heron at 1-844-437-6611 or to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Indication
ZYNRELEF is indicated in adults for instillation to produce postsurgical analgesia for up to 72 hours after soft tissue and orthopedic procedures including foot and ankle, and other procedures in which direct exposure to articular cartilage is avoided.
Limitations of Use: Safety and efficacy have not been established in highly vascular surgeries, such as intrathoracic, large 4 or more level spinal, and head and neck procedures.
Please see full Prescribing Information, including Boxed Warning and updated Warnings and Precautions for serious skin reactions caused by nonsteroidal anti-inflammatory drugs (NSAIDs).
